Abstract
Introduction Mantle-cell lymphoma (MCL) is a B-cell non-Hodgkin Lymphoma (NHL) characterized either by indolent clinical course or highly aggressive and refractory disease with poor prognosis. Drug resistance may be sustained by Tumor Microenvironment, since Tumor-Associated Macrophages (TAM) experienced M2-like polarization mediated by MCL blast cells, which in turn received from immunosuppressive M2-like TAM tumorigenic and survival signals, thus fueling immune evasion (Le, Blood Adv. 2021).
Recently, "Don't Eat Me" signal (DEMs) blockade with anti-CD47 monoclonal Antibody (mAb) showed satisfying results in pretreated NHL, through an increase of phagocytosis by TAM (Advani, NEJM. 2019).
In a preclinical model of solid cancer, CD24 was also demonstrated to be involved in DEMs, showing an improvement of M2-like TAM-mediated phagocytosis in vitro and an increase of survival in vivo by blocking the CD24/Siglec-10 interaction (Barkal, Nature. 2019).
As already shown (Aroldi, Blood. 2021. doi.org/10.1182/blood-2021-154086), CD24 is also upregulated by MCL and the CD24/Siglec-10 blockade was found to improve phagocytosis of human MCL cell lines (huMCL) in an antigen density-dependent manner.
Here, we present an update of our in vitro results of CD24/Siglec-10 DEMs blockade in MCL, exploring the feasibility of combination of DEMs blockade with conventional therapies like anti-CD20 mAb (i.e., Rituximab).
Methods CD24 antigen expression of huMCL, monocytes isolation, in vitro M2-like TAM differentiation, evaluation of CD24-ligand Siglec-10 upregulation in M2-like TAM and methods for co-culture analysis by flow cytometry were previously described (Aroldi, Blood. 2021).
Phagocytosis was measured (flow cytometry) as the number of CD11b+/Carboxyfluorescein succinimidyl ester (CFSE)+ TAM, quantified as a percentage of the total CD11b+ TAM. Each phagocytosis assay was performed in triplicates and phagocytosis was normalized to the highest technical replicate per donor in order to consider raw phagocytic level among donor-derived macrophages.
Phagocytosis assays by fluorescent microscopy was performed by staining huMCL with CFSE and TAM with 4',6-diamidino-2-phenylindole (DAPI). The number of CFSE+ cells within macrophages is counted to obtain the phagocytic index, determined as the number of ingested cells per 100 macrophages (counting at least 200 macrophages per condition).
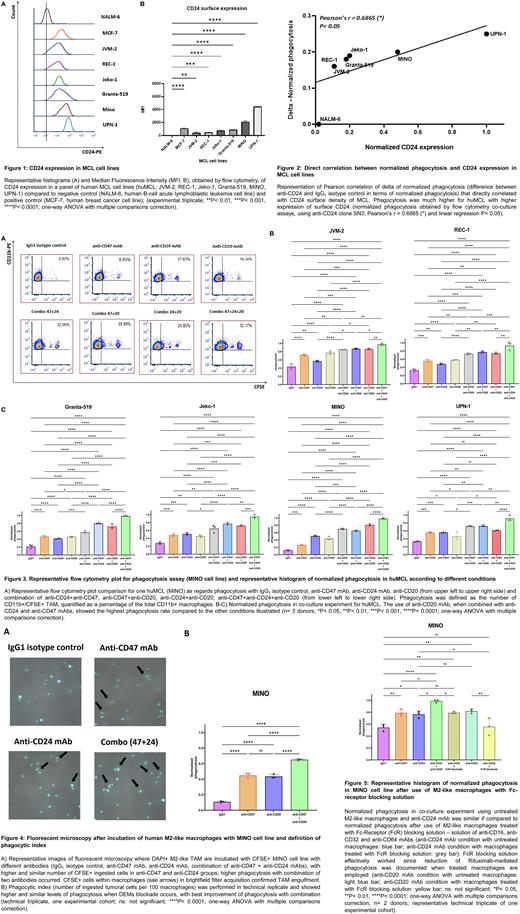
Results CD24 surface expression was checked in an extended panel of huMCL and is represented in Figure 1. Phagocytosis was improved in all huMCL when co-cultured together with M2-like TAM and anti-CD24 mAb (clone SN3), inducing phagocytic activity in an antigen density-dependent manner (Figure 2).
The highest rate of phagocytosis was documented in case of combination of anti-CD24 with anti-CD47 (clone B6H12.2) and anti-CD20 (clone Rituximab) mAbs, compared to the other conditions (Figure 3). Similarly, fluorescent microscopy assays displayed improved phagocytosis when DEMs blockade occurred, showing CFSE+ ingested cells within TAM cytoplasm (Figure 4).
Finally, to demonstrate that the improved phagocytosis by anti-CD24 was due to blockade of DEMs rather than to Fragment crystallizable (Fc)-mediated opsonization, we co-cultured M2-like TAM, pre-treated with Fc-receptors (FcR) blocking mAbs, and MCL, in order to shut down Fc-mediated phagocytosis during co-culture. Phagocytosis produced by anti-CD24 still occurred when FcR-blocked (FcRb) TAM were used, depicting the similar rate of phagocytosis experienced when untreated M2-like TAM were employed. Conversely, phagocytosis elicited by anti-CD20, whose activity is mainly triggered by Fc-mediated opsonization, is quenched when FcRb TAM were used (Figure 5).
Conclusions huMCL turned out to be susceptible to CD24/Siglec-10 DEMs blockade when co-cultured with M2-like TAM. The observed increase of phagocytosis is influenced by CD24 surface density of MCL and is secondary to loss of CD24 signalling rather than to Fc-mediated opsonization.
The combination with another DEMs blockade (i.e., anti-CD47) provided higher levels of phagocytosis when compared to single-agent conditions. The addition of Rituximab caused a further increase in the phagocytosis of huMCL in case of triple combination, thus paving the way for future clinical use.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.